
The meanings of the chemical phrases atomic mass and mass number are not the same. Simply subtract the number of protons, or atomic number, from the mass number to determine how many neutrons an atom has.Īns. The mass number of an element is determined by the number of protons and neutrons added together:Ītomic mass = Mass of protons + Mass of neutrons + Mass of electrons 1 atomic mass unit (amu) is equal to 1.660539040 1024 gramme on this scale.Īns. It is given as a multiple of one-twelfth the mass of the carbon-12 atom, which has an atomic mass of 12 units, 1.992646547 1023 gramme. The amount of matter contained in an atom of an element is called its atomic mass. Inches To Centimeters- Conversion, Chart & FormulaĪns.Differentiation: Equations, Formula, Sum, Product, Quotient And Chain Rule.Nephron- Structure, Function And Diagram.
#PERIODIC TABLE OF ELEMENTS ATOMIC WEIGHT PDF#

Overall atomic masses are determined for naturally occurring isotopes of each element, weighted by the weight of those individual isotopes on Earth, as shown in periodic tables like the one for hydrogen. The amount of example an isotope makes up determines how much it contributes to the normal. The normal of the atomic masses of the seeming plurality of distinct isotopes in an example is the general atomic mass. Because the isotopes of an element have different atomic masses, researchers can determine the element’s overall atomic mass (also known as the atomic weight). In most cases, the atomic mass number is rounded to the next whole number. Carbon-12, for example, is a typical carbon atom with six neutrons and six protons. The absolute mass of a single atom is its atomic mass, which is measured in atomic mass units, or amu. Carbon-12 has 6 protons and 6 neutrons, carbon-13 has 6 protons and 7 neutrons, and carbon-14 has 6 protons and 8 neutrons.
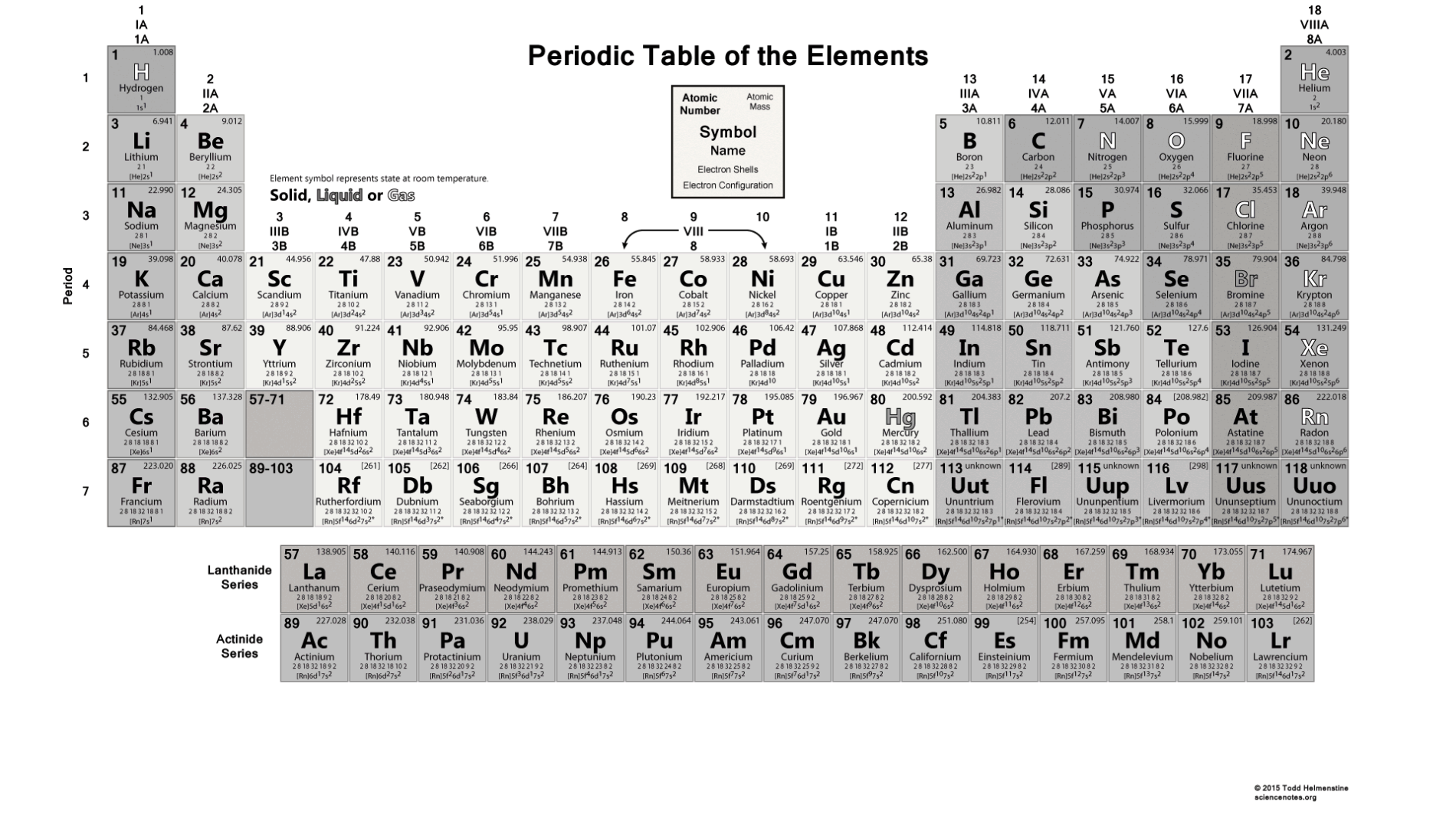
For example, carbon has three isotopes: carbon-12, carbon-13, and carbon-14. Isotopes are atoms of the same element that have different numbers of neutrons.

It is typically expressed in atomic mass units (amu), which are defined as 1/12 of the mass of a carbon-12 atom.The atomic mass of an atom is a weighted average of the masses of the isotopes of that atom. Atomic Mass of Elements 1 to 30 with Symbol and PDFĪtomic mass is the mass of an atom.Atomic Mass of Elements 1 to 20 with Symbols.Atomic Mass of Elements 1 to 30 with Symbols.NCERT Solutions Class 10 Social Science.NCERT Solutions For Statistics Class 11.


 0 kommentar(er)
0 kommentar(er)
